C19 mRNA clinical trial rabbit holes and production disasters. People should demand to see the contracts signed by their idiot governments immediately.
I hope everyone saw the New Year in with as little damage as possible!
Here are some thoughts relating to the progression from the tens of thousands of doses used in clinical trials - the “theory” of the trials - to the massive scale-up to the “practice” of making and distributing billions of doses of the injection across state lines, countries and continents.
Question 1: Are clinical trial participants go home DURING each clinical trial phase? (How long does each clinical trial phase last?)
The reason for the question is that people injected might be carriers of spike proteins that may or may not resemble the spike protein in the SARS-COV2 virus. The Chinese sent over the spike protein code that they had identified which was gleefully copied by big pharma in just a few hours/days with no verification of the validity of the spike protein code by anyone. Hell, we do not even know if the mRNA instructions replicate the original WuFLu spike protein.
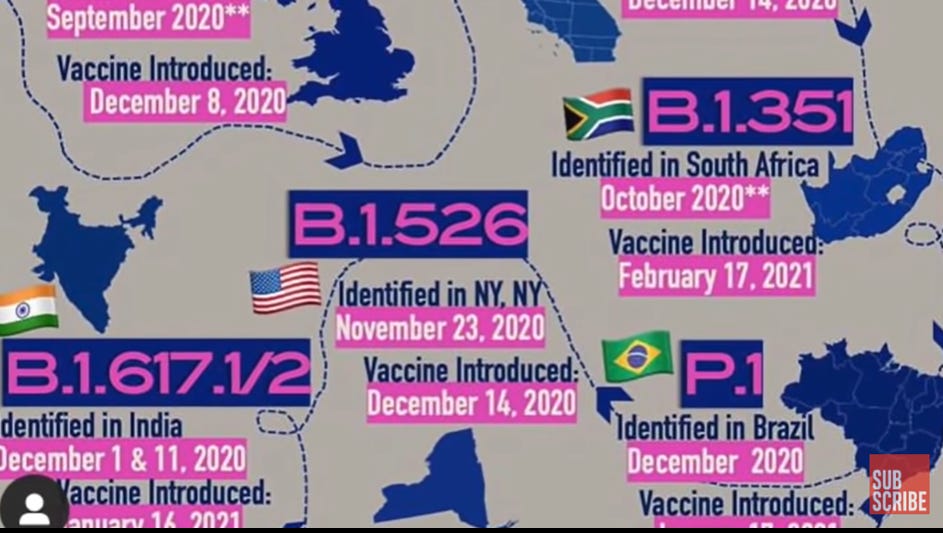
The screen shot below, taken from this video at the 2 minute 12 mark points out the coincidental outbreaks of variants at ALL clinical trial sites. (The video is a moving representation of this snapped image, you can let the video run before and after the mark to get the whole picture).
If a “variant” has an R0 of 6 and 100 people from a trial are wandering around, it would take just 9 days for a billion people to be infected. (6 to the ninth power times 100 people – feel free to correct the maths if faulty!).
Keep in mind that Phase 3 clinical trials around the world had over 40,000 for Pfizer and 30,000 for Moderna (plus thousands more for Astra Zeneca and Johnson and Johnson).
It would not take much for even a fraction of those injected to “shed” their spike protein and infect huge numbers.
I have been unable to see whether clinical trial participants are confined to sterile medical facilities during the entire period of each of the 3 phases of the clinical trials or whether they ar released to the general population after testing and monitoring.
Perhaps this hypothesis can be easily rebutted and it is indeed a rabbit hole with a dead end.
Here’s a few more results from some web surfing.
From here: Clinical trial phases:
“There are three phases of clinical trials that must be completed before a drug is approved by the Food and Drug Administration (FDA).
If a treatment appears safe at the end of a phase 1 clinical trial, it may move forward to a phase 2 clinical trial.
A phase 2 clinical trial is done to see if a treatment is effective. If a drug or treatment is deemed safe in a phase 1 trial and effective in a phase 2 trial, it will then enter a phase 3 clinical trial.
Phase 3 clinical trials often have a larger study population and are done to see if a treatment works better or has fewer side effects than available treatments.”
Phase 1 (after animal trials) involves a small number of people, “..significant and positive results must be found in order to have a human trial approved”..” determine if a drug or treatment is safe, the best dose of a drug, and how it should be given”.
More detail here Phase 2, “..Phase II trials can be subdivided into Phase IIa and Phase IIb; the former is essentially a pilot study involving a limited number (∼50) of participants, whereas the latter will often recruit several hundred epilepsy patients.”
but from the “Clinical Trial Phases” link:
“A phase 2 clinical trial is done to see if a treatment is effective. If a drug or treatment is deemed safe in a phase 1 trial and effective in a phase 2 trial, it will then enter a phase 3 clinical trial.”
“Phase 3 clinical trials often have a larger study population and are done to see if a treatment works better or has fewer side effects than available treatments.”
Here are some analyses of the Phase 3 clinical trials – in short, lies, damned lies and sadistics (sic).
Pfizer’s Phase 3 clinical trials had around 21,900 in each of the injected arm and placebo arm. Analysis of that trial that produced Level 1 Evidence of HARM is here. Here is Sasha Laytpova’s take down of Moderna’s clinical trial processes, with Robert Kennedy of Children’s Health Defence. I note that Dr John Campbell discussed another more recent (and damning) analysis here.
Just to nail the clinical trial methodology here are links to detailed narratives for Phase 1, Phase 2, Phase 3 and Phase 4 trials.
The putcomes of clinical trials? The clinical trials FAILED and caused outbreaks of more and less deadly and infectious variants across the globe (that would not have “broken out” had Ivermectin and HCQ protocols been used instead no C19 by the Fall of 2020). There would have been no loss of life running to millions or and hundreds of millions of “cases” around the world. No need for masking, lockdowns or any other emergency measure. The pandemic would have been over within Trump’s presidency.
Which leads to the second question.
What is the split between deaths and injuries reported to adverse event reporting systems like VAERS and EUDRA from
1) doses that comport with the clinical trial doses,
2) doses that do NOT match the doses used in clinical trials,
3) are a result of poor quality manufacturing (stainless steel shards in the vials, broken lipids, PEG coating, escaped mRNA, altered mRNA etc).
4) also had actual PAST SARS-COV2 from prior periods (maybe compounded by C19 mRNA injections or not caused by C19 mRNA injections at all)
I urge you to watch these two videos (already linked in another article). They relate to the approval of clinical trial data and/or the “scale up” from a few thousand doses to billions upon billions of them.
I doubt there is a single production line anywhere in the world for any product that did not break down or start “deviating from the mean” by a small (or large) margin,
Hang on every word.
The first video (transcript needed!) focuses on the failure of the EMA to engage its (separate and distinct from https://www.bitchute.com/video/1MG7hHpprd9P/the US FDA/CDC) regulatory obligations – with, for example, big pharma saying “you cannot look at this, it is proprietary” and other areas like “the spike protein manufactured by the body in response to mRNA instructions Is 30% bigger than the “natural” spike protein” or no mRNA manufacturing plant ever achieved greater than 85% consistency with the clinical doses. Pr even that no regulatory body anywhere was intellectually capable of proving that the doses they received were consistent with clinical trial does. Plus lots more red flags.
The second video focuses on deadly batches in Australia that could have and should have been pulled from the market because of clear evidence of association with death and adverse events.
Lastly, here is the COUNTRY agreement signed by Slovenia for its Pfizer doses. This is probably a global template per country.
“Selected extract:
“..the Vaccine and materials related to the Vaccine and materials related to the Vaccine, and their components and constituent materials are being rapidly developed..”
Rapidly developed????? Wtf were the clinical trials for?
“The Participating Member State further acknowledges that the long-term effects and efficacy of the Vaccine are not currently known and that there may be adverse effects from the Vaccine that are not currently known.”
Yeah, so sign here and be damned - or more accurately damn your people and go lie to them that the injections are “safe and effective”!!!
Pfizer can change the contents at will with no proofs of any benefit and with no liability for any country that signed an agreement like this.
Thanks to my subscribers so far, don’t forget to subscribe if you are able and willing, or buy me a coffee here if you liked the article! https://ko-fi.com/peterhalligan


Apologies, I thought the Phase III trials are ongoing via the general population, and will end formally in March of 2023?
The rise of variants is working now ;) thanks again.